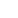Sodium nitrite
Sodium nitrite
| Kayayyaki | |
| Tsarin sinadaran | NaNO3 |
| Molar taro | 84.9947 g/mol |
| Bayyanar | Farin foda |
| Yawan yawa | 2.257 g/cm3, m |
| Wurin narkewa | 308 °C (586 °F; 581 K) |
| Wurin tafasa | 380 °C (716 °F; 653 K) yana rubewa |
| Solubility a cikin ruwa | 73 g/100 ml (0 °C) 91.2 g/100 ml (25 ° C) 180 g/100 ml (100 ° C) |
| Solubility | sosai mai narkewa a cikin ammonia, hydrazine mai narkewa a cikin barasa dan kadan mai narkewa a cikin pyridine insoluble a cikin acetone |
Sodium nitrite (NaNO2) gishiri ne wanda ba a iya gani ba wanda aka samo shi ta hanyar ions nitrite da halayen sodium ions.Sodium nitrite yana da sauƙi mai sauƙi kuma mai narkewa a cikin ruwa da ammonia na ruwa.Maganin ruwan sa shine alkaline, PH yana kusan 9;kuma yana da ɗan narkewa a cikin ƙwayoyin halitta kamar ethanol, methanol da ether.Yana da ƙarfi oxidizer kuma yana da kaddarorin ragewa kuma.Lokacin da aka fallasa zuwa iska, Sodium Nitrite za ta zama oxidized sannu a hankali, kuma ta zama sodium nitrate a saman.Ana fitar da iskar iskar nitrogen dioxide ta Brown a ƙarƙashin raunin acid.Tuntuɓar kwayoyin halitta ko rage wakili zai haifar da fashewa ko konewa, haka kuma, sakewa mai guba da iskar gas na nitrogen oxide.Sodium nitrite kuma yana iya zama oxidized ta hanyar abubuwan da ke haifar da iskar oxygen mai ƙarfi, musamman ammonium gishiri, kamar ammonium nitrate, ammonium persulfate, da sauransu, waɗanda za su iya yin hulɗa tare da juna don samar da zafi mai zafi a yanayin zafi na yau da kullun, wanda ke haifar da abubuwan ƙonewa don ƙonewa.Idan mai zafi zuwa 320 ℃ ko sama, Sodium Nitrite zai rushe zuwa oxygen, nitrogen oxide da sodium oxide.Lokacin tuntuɓar kwayoyin halitta, yana da sauƙin ƙonewa da fashe.
Aikace-aikace:
Binciken Chromatographic: Ana amfani da nazarin ɗigon ruwa don tantance mercury, potassium da chlorate.
Dizotization reagents: Nitrosation reagent;Binciken ƙasa;Ƙaddamar da ƙwayar bilirubin a cikin gwajin aikin hanta.
Wakilin Bleaching don siliki da lilin, wakilin maganin zafi na ƙarfe;mai hana lalata karfe;Maganin guba na Cyanide, reagents na nazari na dakin gwaje-gwaje.A cikin yanki na abinci, ana amfani da shi azaman wakilai na chromophores lokacin sarrafa kayan nama, da kuma magungunan antimicrobial, masu kiyayewa.Hakanan yana da aikace-aikace a cikin bleaching, electroplating da maganin ƙarfe.
Hankalin ajiya: Ya kamata a adana nitrite sodium a cikin ƙananan zafin jiki, bushe da shago mai iska.Ƙofofi da tagogin windows suna matse don hana hasken rana kai tsaye.Ana iya adana shi a cikin hannun jari tare da wasu nitrates ban da ammonium nitrate, amma an raba shi da kwayoyin halitta, kwayoyin da ba su ƙonewa, rage wakili da tushen wuta.